THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.
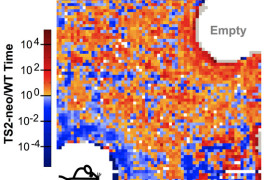
Cold shoulder: Mice carrying the Timothy syndrome mutation tend to spend more time near an empty cage (red areas), whereas controls gather near a cage holding another mouse (blue areas) P. Bader.
Researchers have created the first mouse model of Timothy syndrome, a rare genetic disorder that causes heart defects and autism. Researchers first presented the findings, published 30 August in the Proceedings of the National Academy of Sciences1, at the Society for Neuroscience annual meeting last November.
Individuals with the syndrome carry mutations in the CACNA1C gene, which controls the flow of calcium ions in and out of cells. The mutant mice, which express the defective gene at one-third the normal level, show mild social deficits, repetitive behavior and shorter vocalizations than controls.
“We have here all three elements of the classic autistic triad: the social behaviors, the repetitive behaviors and the communications difficulty,” says lead investigator Richard Tsien, professor of molecular and genetic medicine at Stanford University in Palo Alto, California.
There are only a few dozen reported cases of the Timothy syndrome mutation, which causes an unchecked influx of calcium ions in cells. This disturbs heart muscle contractions, sometimes leading to fatally slow heartbeats. It also upsets the firing patterns of neurons. About three-quarters of individuals with Timothy syndrome also show symptoms of autism.
Although the mutation is rare, the autism community is paying attention to Timothy syndrome because CACNA1C has much in common with other autism candidate genes. It encodes a protein called the L-type channel that sits at the synapse and regulates calcium signaling, which in turn controls gene expression.
Most of the autism genes identified to date fall into one of two categories: They either affect the synapse, the junction between neurons, or they’re involved in the translation of DNA into protein. “The L-type channel just sits beautifully at the watershed between these two camps,” Tsien says.
Cassette players:
Researchers’ first attempts at making a mouse model of Timothy syndrome failed because animals expressing the mutation at normal levels did not survive.
Tsien’s group cleared this hurdle using a trick of molecular engineering. One way to create a mutant mouse is to insert the desired genetic mutation along with a so-called neomycin cassette, a string of DNA that codes for resistance to an antibiotic called neomycin. By later exposing the cells to the antibiotic, the researchers can select for those that successfully received the target mutation.
Once this is done, researchers typically remove the cassette from the transformed cells. In this study, however, they left it in. The cassette interferes with the transcription of nearby genes, ultimately dampening their expression. In this case, that meant that the mutant mice only show one-third the normal level of CACNA1C expression — not enough to be lethal, but apparently enough to cause behaviors reminiscent of autism.
The most striking feature, according to the researchers, is the mutants’ strong resistance to change. In a test called the Y maze, mice typically swim along a straight path and are taught to make a turn in order to find a dry platform. They are first trained to go to one side, but then the platform is switched to the other side.
Normal mice learn to switch to the correct side within a few trials. Many of the Timothy mutants, however, steadfastly insist on taking the old route, even after 25 trials (see video). The researchers tried putting up a plastic partition to prevent the mice from making the wrong turn. “Even then, they would bump their head against the block,” says Patrick Bader, a trained psychiatrist and postdoctoral fellow in Tsien’s lab. “It was very, very exciting to see that.”
The mice show similar problems during a memory test in which they are trained to associate a certain sound with a mild electrical shock. Once they learn the association, mice respond to the tone, even when not accompanied by a shock, by freezing in fear. When they hear the sound more than two weeks after learning the initial association, however, normal mice are less afraid, freezing for much shorter lengths of time, whereas the mutants tend to stay frozen.
“Our hypothesis is that the Timothy syndrome mice might have more problems un-learning something,” Bader says.
Subtle effects:
The mutants also have mild social deficits, as determined by a new behavioral test developed in collaboration with Mehrdad Shamloo, also of Stanford.
In the four-hour test, mice roam in a dark cage that has two unfamiliar objects: an empty pencil cup, and a cup holding another mouse. After the mice acclimate to the cage for two hours, researchers use an infrared camera to measure where the mice spend their time. In the second two hours of the test, normal mice tend to spend more time with the other mouse, whereas mutants veer toward the empty cup.
“I really like this test, and I’m thinking to myself very selfishly, I wouldn’t mind using it,” notes Valerie Bolivar, director of the Mouse Behavioral Phenotype Analysis Core at the Wadsworth Center, New York State Department of Health. The test is useful because it’s akin to the long-term social interactions of people with autism, she says.
“If we want to apply this to autistic individuals, we’d want to consider the social behavior in the home, with people they know and spend time with, not strangers,” Bolivar says.
However, it’s difficult to say whether this mimics the social behaviors of people with Timothy syndrome, researchers say, because the disease is so rare and each individual has a unique mix of symptoms and traits.
“The psychiatric manifestations of the disease are often very subtle,” notes Silvia Priori, who was part of the team that first defined the syndrome in 2004 and now treats eight individuals with the disease2.
What’s more, the mutation’s other effects — such as webbed fingers, fainting and a gag reflex — could in themselves lead to strained social interactions.
“The fascinating part of this disease is that, maybe because calcium is such an important mediator of embryological development in different tissues, these patients seem to have a uniquely complex phenotype,” says Priori, professor of medicine at New York University.
The new mutant mice do not have heart defects, perhaps because not enough of the faulty gene is expressed, or because mouse hearts beat much faster than human hearts do. “It’s a shame that the model cannot be used to study the cardiac phenotype,” Priori says.
Still, the mutants could test whether drugs that act on calcium channels — such as calcium blockers, which are commonly used to treat high blood pressure — have a beneficial effect on the animals’ social and communicative defects.
“It could be a good tool to try to shed some light on the psychological and developmental abnormalities in these patients,” Priori says.
By joining the discussion, you agree to our privacy policy.