Social difficulties and deficits in inhibitory signals across the hippocampus characterize mice lacking one copy of the autism-associated gene DYRK1A, a new study finds. Activating inhibitory neurons in this brain region can improve the animals’ social skills, the study also shows.
The findings reveal the neuronal circuits underlying some difficulties related to mutations in DRYK1A, which have been linked to intellectual disability, a smaller-than-average head, seizures and autism.
Previous work has shown that mutations in DYRK1A influence social cognition, but the underlying circuit mechanisms were unclear, says lead investigator Amar Sahay, associate professor of psychiatry at the Center for Regenerative Medicine at Massachusetts General Hospital in Boston.
Sahay’s findings suggest that targeting molecules and circuits that are downstream of the gene can reverse DYRK1A-associated social difficulties, even in adulthood. “I don’t think we will ever be able to restore the arc of typical development,” Sahay says. “Therefore, identifying opportunities for intervention in later life affords tremendous hope for new therapeutic interventions.”
The study has the merit of tackling social recognition from different angles, says Azahara Oliva, assistant professor of neurobiology and behavior at Cornell University, who wasn’t involved in the work. The researchers, she says, “have narrowed down not only where to look in the brain, but also what to look for — they even suggest how to restore [social recognition] in pathological conditions.”
T
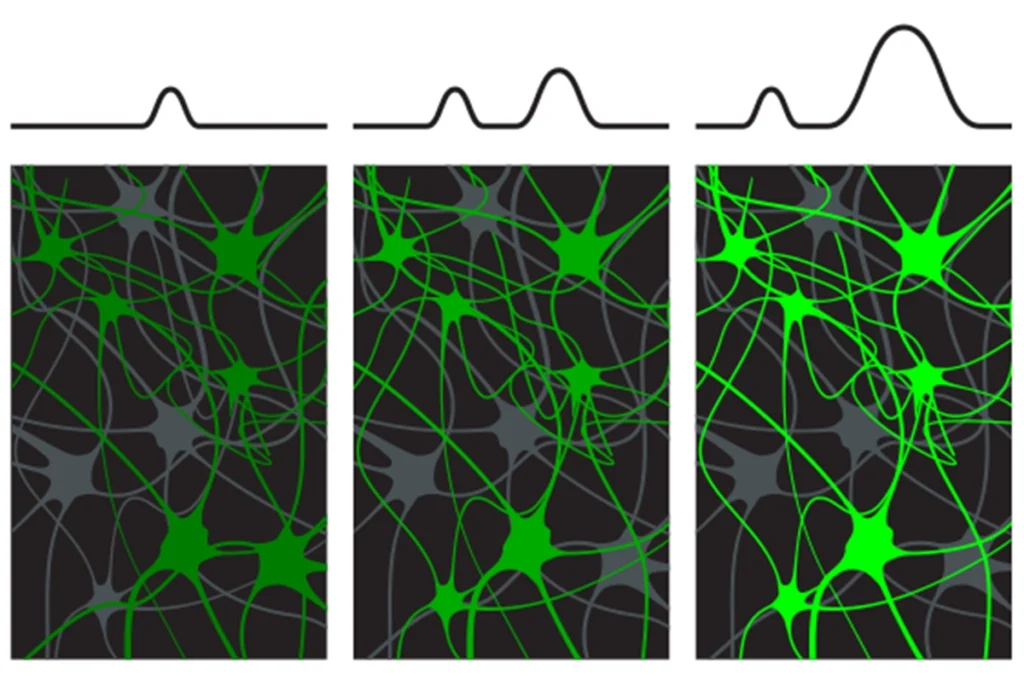
he learning process modifies inhibitory circuitry in three adjoining parts of the hippocampus — CA2, CA3 and the dentate gyrus — Sahay and his colleagues reported in 2018 and 2022. In response to experience, granule cells in the dentate gyrus increase their number of synapses with parvalbumin neurons, a type of inhibitory neuron. These neurons, in turn, increase their inhibitory contacts with downstream excitatory neurons in CA2 and CA3.“The result is that in response to experience, there is an increase in feedforward inhibition of the CA2 and CA3 cells,” Sahay says. This inhibition is crucial for the simultaneous firing of neurons in groups, or “ensembles,” and the generation of brain oscillations that have been linked to learning and memory, he says.
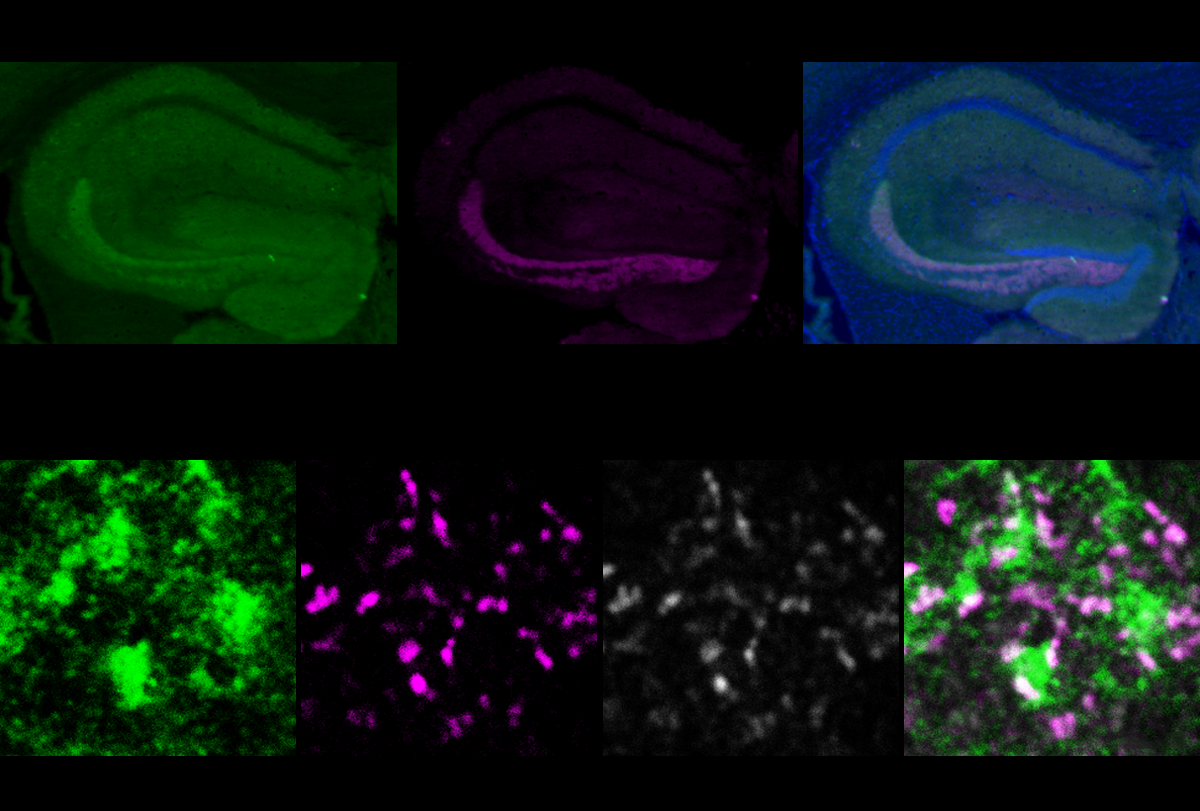
“Feedforward” inhibition of excitatory neurons in CA2 and CA3 also occurs when mice are exposed to an unfamiliar mouse, according to the new study. This inhibition decreased after the scientists deleted DYRK1A only in the granule cells of adult mice. Unlike controls, these mice did not prefer to interact with a new mouse over an empty cup, indicating difficulties in the early stages of social recognition — often referred to as sociability. Similar circuitry alterations and social difficulties turned up in mice lacking a copy of DYRK1A throughout the body.
Further experiments implicated a protein called ABLIM3, which is expressed only in the granule cell’s axon. ABLIM3 levels typically decrease in response to experience, enabling feedforward inhibition, but they failed to do so in DYRK1A mice.
Dampening ABLIM3 in the granule cells of DYRK1A mice rescued feedforward inhibition and eased the animals’ social-recognition difficulties.
DYRK1A is likely “either downregulating or inactivating ABLIM3, and this results in an increase in feedforward inhibition,” Sahay says.
Activating parvalbumin neurons in DYRK1A mice also improved the animals’ social recognition, the researchers found. The findings were published 4 October in Neuron.
T
he findings bolster the hypothesis that an imbalance between inhibitory and excitatory signals in the brain may underlie some autism traits. “People have been thinking about excitation/inhibition balance in autism for a long time,” says Vikaas Sohal, associate professor of psychiatry and behavioral sciences at the University of California, San Francisco, who did not take part in the study. “What’s new here is seeing how [the loss of DRYK1A] in an excitatory cell type ends up affecting inhibition one or two synapses downstream.”Showing that social difficulties can be reversed in adulthood is important, and doing so by targeting ABLIM3 or parvalbumin neurons suggests that researchers should look in more detail at the consequences of the loss of DYRK1A in the function of downstream molecules and neuronal circuits, Sohal says.
Among DYRK1A’s downstream molecules, ABLIM3 stands out, and the study raises interesting questions about its role in granule cells in the hippocampus, says Jessica Cardin, associate professor of neuroscience at Yale University. ABLIM3 binds actin — an essential cytoskeletal protein, and alterations in the neuronal cytoskeleton have been associated with autism. The findings, Cardin says, “suggest that there is some potential for structural reorganization relating to feedforward inhibition.”
Although the study provides a window into potential therapeutic targets, before moving to the clinic researchers should investigate whether DYRK1A mutations have similar effects in different areas of the brain, she says. “There may be other pieces of the puzzle remaining to be further examined.”