Some mutations that disable SCN2A, one of the genes most strongly linked to autism, can unexpectedly make neurons hyperexcitable, a study in mice shows. The findings may help explain why a sizeable proportion of autistic children with mutations in SCN2A experience epileptic seizures.
SCN2A codes for Nav1.2, a sodium ion channel that helps propagate electrical impulses through neurons in the brain. Mutations that increase the channel’s activity are associated with seizures during infancy, previous research shows, whereas ‘loss-of-function’ mutations — those that disrupt the channel’s activity — are linked to intellectual disability and autism.
But about a quarter of people with loss-of-function mutations in a single copy of SCN2A also develop epilepsy, presenting researchers with a puzzle: Seizures typically result from an overabundance of excitatory signals; mutations that block or reduce the activity of the sodium channel would be expected to silence that signaling.
“You’ve dampened the excitatory drive on the neuronal network, so why would neurons become more excitable? It just didn’t make any sense,” says lead researcher Kevin Bender, associate professor of neurology at the University of California, San Francisco.
The new study uncovers the cellular mechanisms that could explain this apparent paradox, Bender says.
Channel activity:
Bender and his colleagues previously showed that in mice that have only one functional copy of SCN2A, excitatory cells called pyramidal neurons are not hyperexcitable. Instead of having seizures, the rodents have slower-than-average brain signaling and immature synapses.
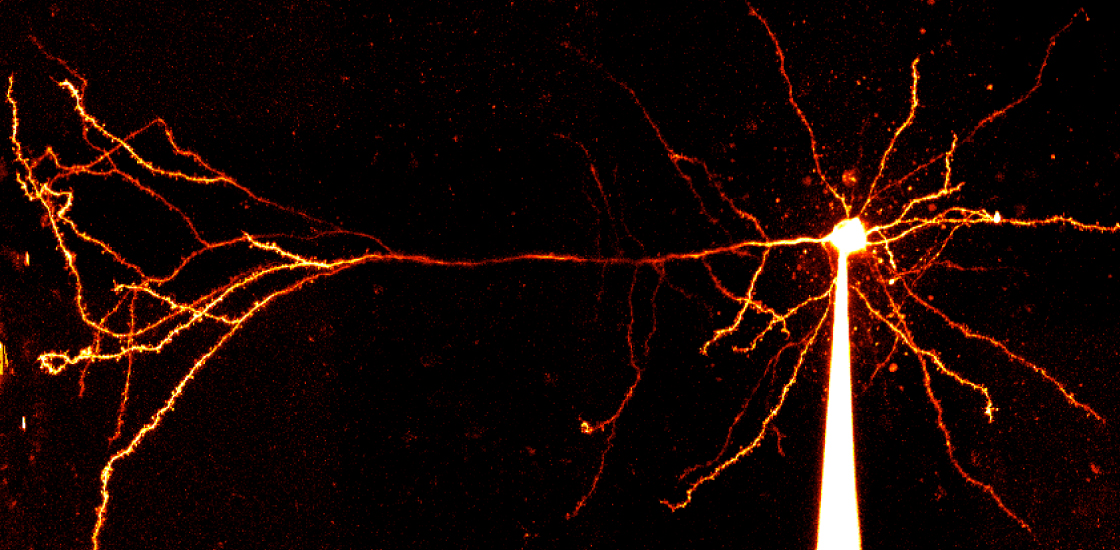
This time around, the team analyzed mice lacking both copies of SCN2A in pyramidal cells of the prefrontal cortex, a brain region involved in social behavior and other activities associated with autism. The neurons were hyperexcitable and fired frequent bursts of action potentials — the electrical spikes that transmit signals along neuronal projections, the researchers found.
Further experiments indicated that in the absence of SCN2A, the cell’s action potentials aren’t large enough to activate voltage-gated potassium ion channels. These channels typically open after a neuron fires so that potassium ions can flow out of the cell, helping to turn the neuron off. Losing SCN2A, though, prevents the cells from returning to an ‘off’ state, making them hyperexcitable and raising the risk of seizures, Bender says.
Losing more than 50 percent of Nav1.2 activity dramatically increases the neurons’ excitability, additional computer simulations suggested. This threshold could explain why only some children with mutations in one copy of SCN2A are prone to seizures.
“These kids live at a cliff edge of seizure susceptibility, and if they have anything in their genome or environment that makes them more likely to have seizures, then they might have seizures,” Bender says. The findings were published this week in Cell Reports.
Restoring balance:
The new study provides convincing evidence for the link between SCN2A loss and epilepsy, says Jun Hee Kim, associate professor of cellular and integrative physiology at the University of Texas Health Science Center in San Antonio. The interplay between sodium and potassium channels “makes a lot of sense,” she says.
In another study in mice published in the same journal this week, researchers found that substantially lowering SCN2A protein levels triggers a compensatory downregulation of potassium channels. This shift results in hyperexcitable neurons in the cortex and the striatum, which controls movement and other behaviors and is implicated in autism.
The findings demonstrate why ion channels shouldn’t be studied in isolation, says Anastasios Tzingounis, professor of physiology and neurobiology at the University of Connecticut, who was not involved in the new study. Instead, researchers should investigate how mutations in one channel influence the activity of others.
Human development takes much longer than mouse development, Tzingounis cautions. In future studies, researchers should take this into account when trying to explain how the loss of SCN2A could lead to epilepsy in people, he says.
But the new study proposes a mechanism that can be tested, Tzingounis says. “Most studies don’t.”
The findings also hint at a testable treatment, says Bruce Bean, professor of neurobiology at Harvard Medical School, who was not involved in the work. Treating children missing a copy of SCN2A with standard sodium channel inhibitors would be counterproductive, he says, but modulating the activity of specific potassium channels could help to control their seizures.
What makes some children with SCN2A mutations more susceptible to seizures remains unclear, Bean notes, but comparing genomic analyses of people who develop epilepsy with those of people who don’t could be revealing.