Repurposed electronics lens spies neurons across entire mouse brain
When combined with tissue-inflation methods, the microscope can image axons without the need for tissue slicing, the researchers say.
By bloating brain samples and imaging them with a powerful microscope, researchers can reconstruct neurons across the entire mouse brain, according to a new preprint. The technique could help scientists uncover the neural circuits responsible for complex behaviors, as well as the pathways that are altered in neurological conditions.
Tracking axons can help scientists understand how individual neurons and brain areas communicate over long distances. But tracing their path through the brain is tricky, says study investigator Adam Glaser, senior scientist at the Allen Institute for Neural Dynamics in Seattle, Washington. Axons, which are capable of spanning the entire brain, can be less than a micrometer in diameter, so mapping their route requires detailed imaging, he says.
One existing approach involves a microscope that slices off an ultra-thin section of the brain and then scans it, repeating the process about 20,000 times to capture the entire mouse brain. Scientists then blend the images together to form a 3D reconstruction of neuronal pathways.
But the process takes several days and is therefore more prone to complications — bubbles forming on the lens, say — than faster techniques, Glaser says.
And slicing can distort the edges of the image, making it “challenging or impossible” to stitch them back together, says Paul Tillberg, principal scientist at the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Virginia, who was not involved in the study. “This is particularly an issue when reconstructing brain-wide axonal projections, where a single point of confusion can misalign an entire axonal arbor to the wrong neuron,” he says.
I
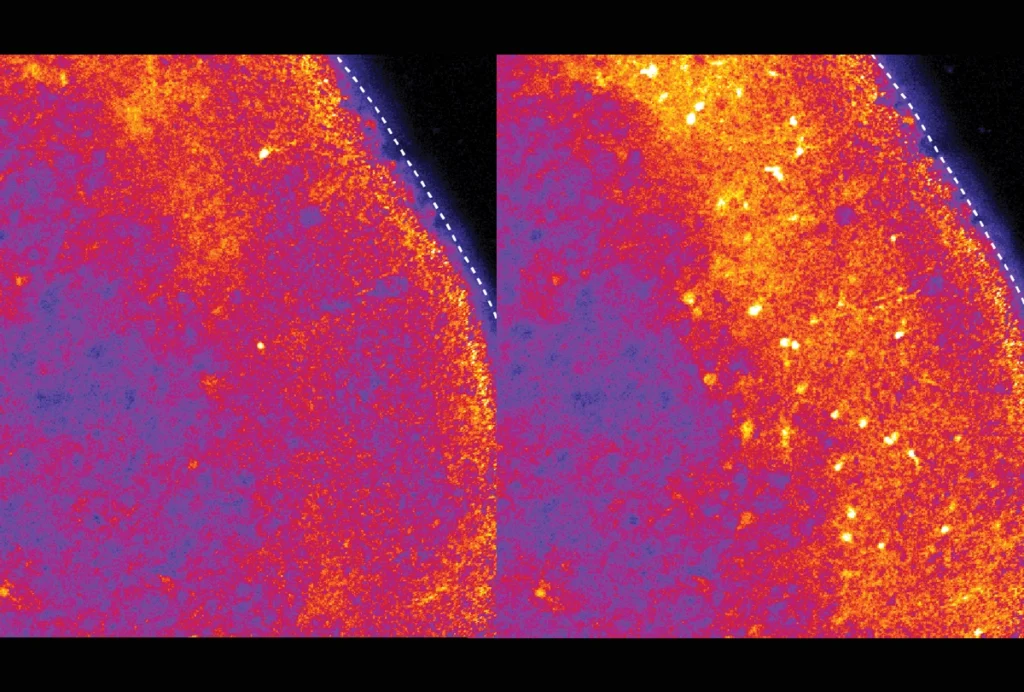
The tool has a field of view 100 times larger, and can image samples 10 times thicker, than objective lenses currently used in scientific research. This allows researchers to scan a mouse brain in just 15 image stacks, without the need to section the tissue and realign hundreds of scans.
But the microscope doesn’t have a high enough resolution to image structures as delicate as axons. To get around this, the team harnessed a technique called expansion microscopy. The method uses a water-absorbing gel to swell tissue, creating space between molecules to increase resolution without damaging cell structures. Because current protocols are optimized for thin samples, the researchers tweaked the method to expand the entire brain.
Tissue expansion has been previously used in combination with light sheet imaging to scan the intact brain of a fruit fly. But the new development goes further, using the technique to image an expanded mouse brain, says Edward Boyden, professor of neurotechnology at the Massachusetts Institute of Technology, whose lab developed expansion microscopy but is not involved in the current work. “They’re able to image the whole darn thing. It’s pretty cool,” he says.
Glaser and his colleagues call the combined technique “expansion-assisted selective plane illumination microscopy,” or ExA-SPIM. The findings were posted on bioRxiv on 27 June.
“[It’s] a very exciting example of creative, interdisciplinary thinking,” Tillberg says.
“This is impressive work,” agrees Joshua Lillvis, research scientist at the Janelia Research Campus, who was not involved in the study. ExA-SPIM may help characterize neural circuits and how they differ between people or are affected by certain brain conditions, Lillvis says.
T
The team repeated the procedure with a centimeter-sized chunk of macaque monkey brain, tracing the axonal projections of fluorescently labelled motor neurons. The process involved less slicing than previous methods and the whole brain could be imaged in about four days, the researchers estimate.
The tool also successfully tracked the long axons in a thin slice of human neocortex, which is involved in conscious thought and language. The feat lays the foundation for mapping neurons in thicker sections, the researchers say.
But the entire human brain, particularly when it is expanded, would be too bulky to image without slicing. The team plans to redesign the microscope to handle even larger samples, Glaser says. They also aim to improve the system’s sensitivity to weaker signals, enabling the detection of individual molecules as well as entire cells.
What’s more, the researchers plan to help other groups build their own microscopes by offering practical support and making the software more user-friendly, Glaser says.
Explore more from The Transmitter
RNA drug corrects calcium signaling in chimeric model of Timothy syndrome