Five different psychedelic drugs share the ability to restore the mature brain to a developmental state during which it can more easily adjust its connections and learn new information, a new study suggests.
Mice treated with the psychedelics were better able to learn from social experiences and showed changes in the brain’s reward center, the researchers found.
The results hint at a common mechanism to account for psychedelics’ “remarkable therapeutic effects,” says lead investigator Gül Dölen, associate professor of neuroscience at Johns Hopkins University in Baltimore, Maryland.
Pinpointing a shared mechanism could accelerate drug design for mental health conditions and shed light on their pathophysiology, says Gabriella Gobbi, professor of psychiatry at McGill University in Montreal, Canada, who was not involved in the work.
But other scientists caution that more work is needed before the findings are translated to people.
Dölen’s group previously uncovered a “critical period” — a window of brain development during which brain circuits are particularly elastic — for social-reward learning in mice. The critical period closes by adulthood but can be restored by treating the mice with the psychedelic drug MDMA, also known as ecstasy, the researchers found.
Yet it was unclear whether the critical period reopens due to the drug’s pro-social effects or the altered state of consciousness it induces. If the latter, all psychedelics — even those that don’t increase sociability — should produce the same effect, Dölen says.
I
n the new work, the researchers treated mice with MDMA or one of four other psychedelic drugs not known to enhance sociability: psilocybin, LSD, ketamine or ibogaine.The mice had previously learned to associate one type of bedding with a social situation and another type with being alone. And given the choice between the two types of bedding, juvenile mice favored the one from the social environment, whereas adults preferred the solitary one.
But adult mice treated with any of the psychedelics opted for the social bedding, hinting that the drugs reopened the critical period and made the mice more sensitive to social rewards, Dölen says.
Psychedelics that produce a longer “trip” — including LSD and ibogaine — opened the critical period for longer than the drugs with briefer mind-altering effects, the researchers found.
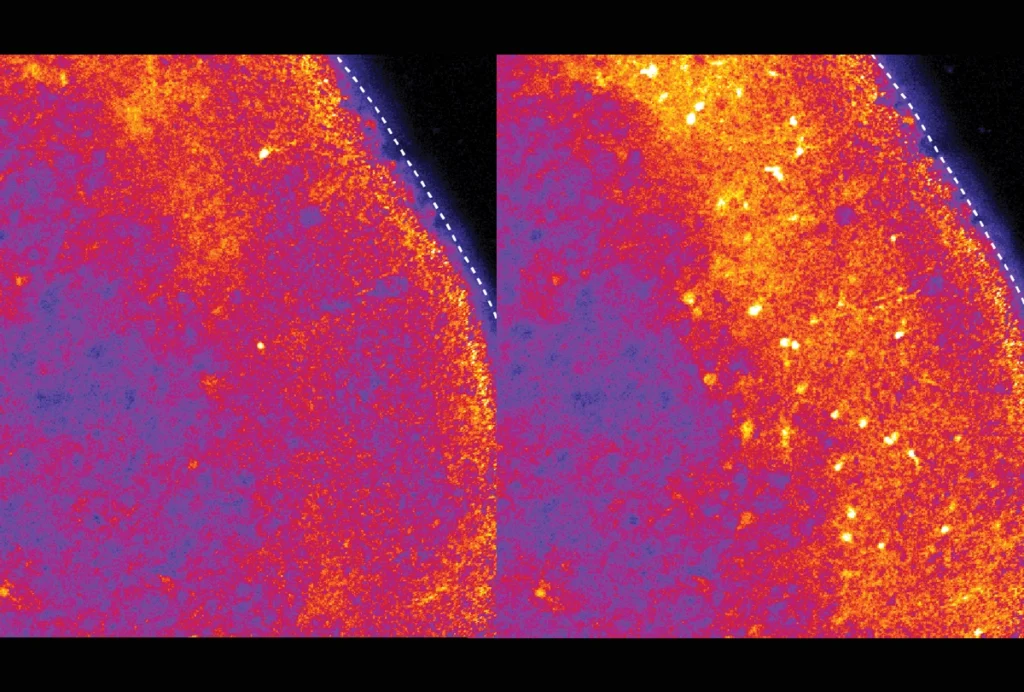
The team then bathed brain slices from the mice in a solution containing oxytocin, a hormone that adjusts neuronal wiring in the brain’s reward center. Neurons in slices from mice given psychedelics were more receptive to oxytocin than those in slices from control mice, suggesting enhanced elasticity of neuronal connections.
Analyses of RNA extracted from mice during and after a drug-induced critical period pinpointed 65 genes that show altered expression between the two states. Of these, many are involved in the remodeling of the extracellular matrix, the supportive mesh between neurons. Matrix degradation may be the mechanism by which psychedelics promote plasticity, despite binding to different targets, the researchers say.
The drugs themselves may not increase synaptic plasticity but rather set the stage for some other stimulus to promote plasticity, Dölen says. This highlights the importance of “set and setting” and could explain why taking drugs at a party versus with a therapist has different outcomes, she adds.
The findings were published 14 June in Nature.
Psychedelics could be used to try to increase the efficacy of treatments for neurodevelopmental conditions, Dölen says. Some of these drugs appeared promising in juvenile mice but later failed in human clinical trials, possibly because the relevant critical period had closed, she says. Administering drugs in combination with psychedelics could help people learn from their social environment as they would during typical development, she says.
“There is a lot of interest for the possible therapeutic use of psychedelics for neurodevelopmental disorders,” Gobbi says. But given their heterogeneous nature, the drugs are unlikely to benefit all, she says.
And first, “much more research is needed,” says Clinton Canal, assistant professor of pharmaceutical sciences at Mercer University in Atlanta, Georgia, who was not involved in the study. It’s unclear whether people have the same critical period for social learning that mice do. And psychedelics would need to be tested in mouse models of neurodevelopmental conditions, as these mice might already have disruptions in their extracellular matrix, he says.
What’s more, psychoactive drugs can produce widely different experiences depending on the individual’s mindset and the environment. What appears to be a positive effect in mice may not translate to people, Canal says.
Dölen’s team is now investigating whether psychedelics could reopen other critical periods, including those involved in vision or motor learning. The drugs may help to restore eyesight following cataract removal, or aid stroke recovery, she says.