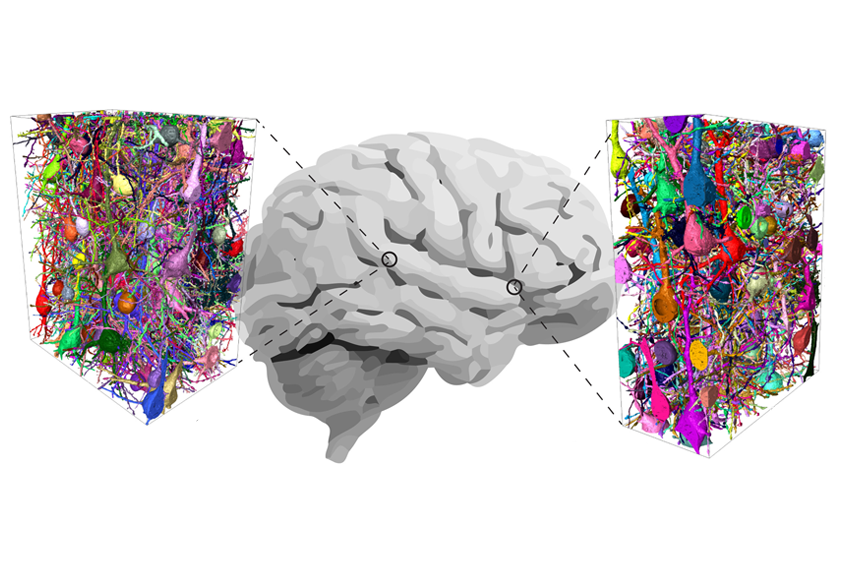
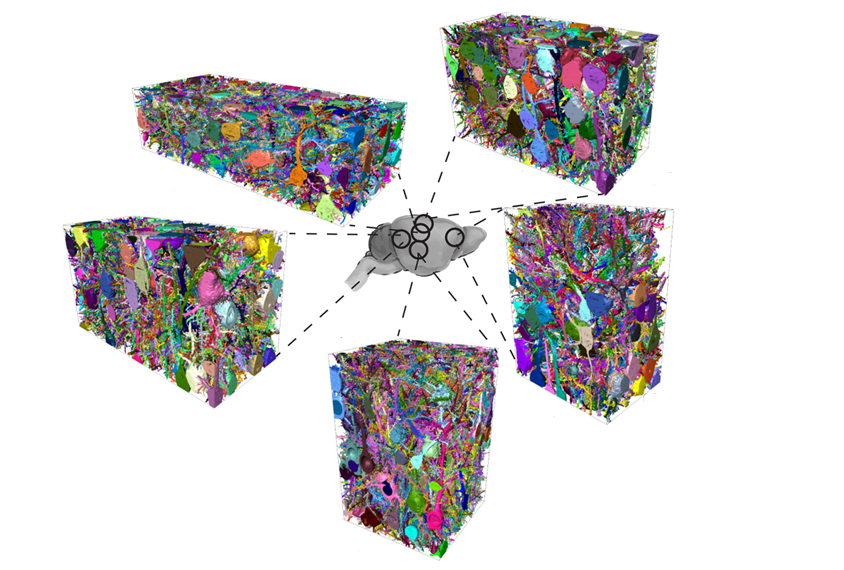
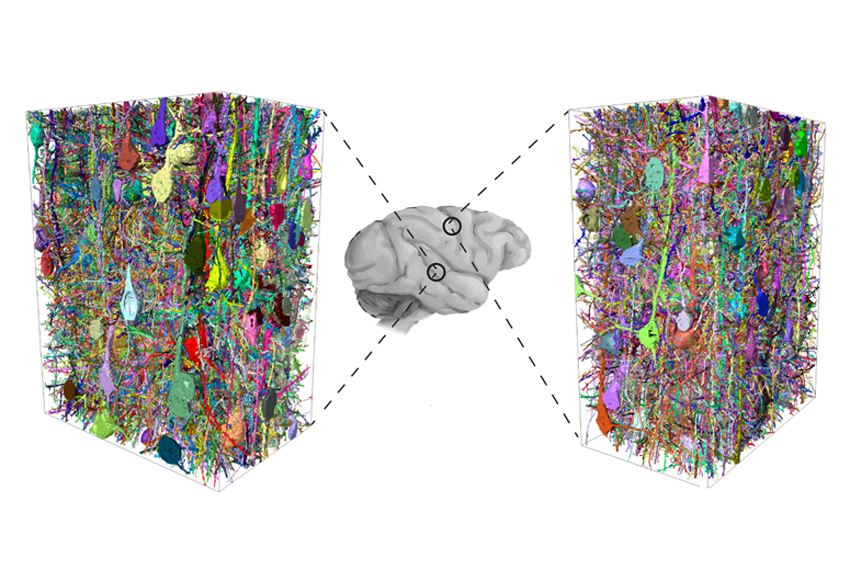
Mouse brains are not tiny human brains.
That may be obvious — and was even the subject of a recent New Yorker spoof. But Moritz Helmstaedter says the fact bears serious consideration because, despite a multitude of clear behavioral differences between mice and people, the neural underpinnings of those differences remain murky.
“That has stunned me ever since med school,” says Helmstaedter, director of the connectomics department at the Max Planck Institute for Brain Research in Frankfurt, Germany. “We had no good idea about what’s really different” between the two species’ brains, he says. “At the level of the circuitry, [they] were stunningly similar.”
To get a better handle on the situation, he and his colleagues mapped the human and mouse connectomes — the complete set of connections between all neurons — in pieces of brain tissue about the size of a fine grain of sand.
People, that comparison revealed, have nearly three times as many inhibitory interneurons as mice do. In fact, the cells make up about 30 percent of the neurons in the human brain, compared with only 12 percent in a mouse’s. The team reported the findings in Science in June.
Spectrum spoke to Helmstaedter about his new work and what the results mean for autism researchers who study inhibition in mice.
This interview has been edited for length and clarity.
Spectrum: What did you think when you saw your first results?
Moritz Helmstaedter: The first thing was to say, “Wow! If there’s three times the amount of inhibition … holy … !” That may mean that the whole inhibition-excitation balance that is such an important topic, especially in pathology-related research fields, is dramatically shifted.
But once we started looking at the complete mapping of all synapses, we could see that other things change at the same time — in particular, the way that axons of excitatory cells innervate other cells.
S: What does that tell you?
MH: Excitatory neurons are the majority of the cells in the brain — around 70 percent of the neurons in humans and around 85 percent in mice. So whenever we talk about inhibition-excitation balance, we primarily mean that excitatory neurons — usually a type called pyramidal cells — are kept from over-excitation and are kept from total silence.
We wanted to see if three times the inhibition means three times the inhibition onto excitatory pyramidal cells. And by carefully measuring, we could see that that was not the case: The excitatory cells in the human brain are not receiving more inhibition than they are in the mouse brain. Which, in a way, was a moment of relief. Because as I just mentioned, for all of these models of psychopathology, this notion of balance between excitation and inhibition was maintained.