Uncertainty and excitement surround one company’s cell therapy for epilepsy
After 10 years of work, Neurona may have the data to quiet its skeptics. But its ongoing clinical trial will be the ultimate test.
About once a month throughout 2012, researchers from four labs at the University of California, San Francisco filed into a conference room in the early morning to hear the latest news on their ambitious project.
The heads of the labs — Arturo Alvarez-Buylla, Scott Baraban, Arnold Kriegstein and John Rubenstein — had formed the company Neurona Therapeutics in 2008 to develop a new approach to treating neurological conditions, using cell therapy. Their goal was to transplant inhibitory interneurons into people’s nervous systems to treat the overexcited circuits that can give rise to conditions such as epilepsy, Alzheimer’s disease and neuropathic pain.
These meetings had grown tense as researchers within the four groups struggled to see eye to eye on data interpretation, particularly when Cory Nicholas, then a postdoctoral researcher in Kriegstein’s lab, presented his protocol for producing the inhibitory interneurons.
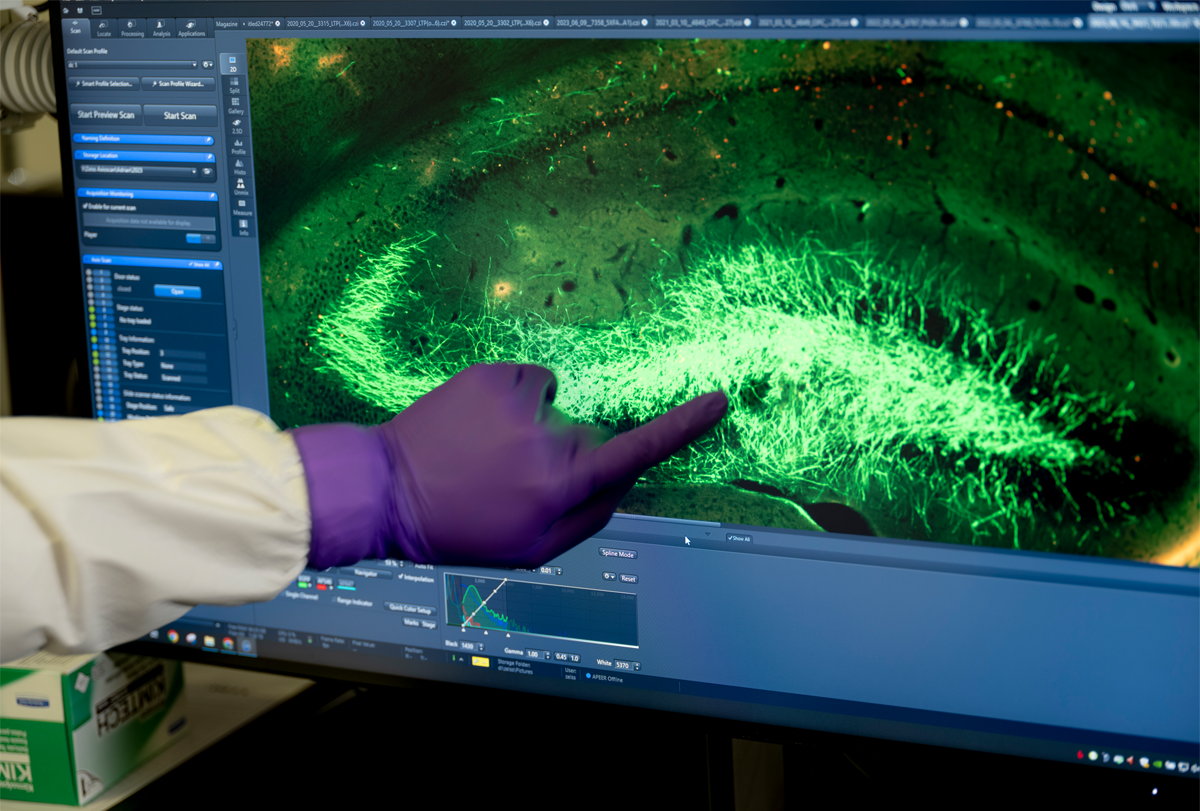
On those days, Nicholas would open his slide deck to reveal images of fluorescent cells on a large screen behind him — red and white branching blobs against a black background. Then the questions would come from Baraban’s group. Why do some of the cells seem to be the wrong kind? Are they forming tumors? Can we see the neighboring images?
There were some in the audience who thought they were being shown only the best images, says Joy Sebe, a postdoc in Baraban’s lab at the time. Nicholas, for his part, says he welcomed the questioning — in science, he says, “your job is to challenge” — and Daniel Vogt, who was a postdoc Rubenstein’s lab at the time, says the back and forth was simply part of the scientific process.
But Sebe believed there was more to it. She did not feel that questions were being answered adequately, she says. And she and some of her colleagues sensed a budding conflict between building a case for the company and interrogating the science.
In the 11 years following those contentious meetings, Baraban parted ways with the group, criticized the group’s published work in multiple reviews and started his own company. Nicholas was officially named Neurona’s chief executive officer, and the company raised $165 million in venture capital funding. And, most importantly, just last year the company began injecting its inhibitory cells, now called NRTX-1001, into people’s brains as part of a clinical trial on epilepsy.
The trial’s preliminary results have been striking, say some observers in the field: The first two participants, who had each been averaging more than a dozen seizures per month, saw a more than 90 percent reduction in the three months after being treated. If those results hold up, NRTX-1001 could be an important new treatment option for people with severe epilepsy, says James McNamara, professor of neuroscience at Duke University in Durham, North Carolina, who is not involved with Neurona.
Today, Nicholas and his Neurona colleagues published their first paper since the company officially launched. It fills in the gaps on the company’s approach and details the research that went into producing NRTX-1001. But even with the new paper, there are still questions about the cells that go into the treatment.
“I do think there should be no doubt if you’re going to put this into people’s brains,” Sebe says.
W
MGE-derived cells have some unusual properties that make them attractive for therapeutic research. For one, immature cells from the MGE disperse throughout the cortex more extensively than do those from other nearby structures, and they maintain that migratory ability when transplanted into the brains of adult mice, rather than forming clumps of cells as other transplanted cells do, Rubenstein says. Grafted into a young mouse’s brain, the cells become mature interneurons that shape the activity of existing circuits — just as they do during embryonic development.
In 2009, Alvarez-Buylla, Baraban and Rubenstein’s labs showed that transplanting mouse MGE cells into the brains of mice that model epilepsy seemed to quell seizures. (Later work showed the same approach to be successful in a different mouse model of epilepsy.)
The problem was that treating people requires human cells, and using human MGE cells, which come from aborted fetal tissue, is ethically and logistically fraught. To avoid this, Nicholas was tasked with using human pluripotent stem cells to produce MGE-like cells. With his stem cell research background and his entrepreneurial spirit, he was eager to make headway.
The goal of this new approach was to coax the cells to develop into the classes of inhibitory interneurons produced by the MGE — somatostatin and parvalbumin-positive neurons — the kind that had proved therapeutic in preclinical trials. The work was challenging, but Nicholas and his colleagues made steady progress, ultimately creating MGE-like progenitor cells, which then developed into inhibitory interneurons. They published the work in Cell Stem Cell in 2013.
Not everyone was convinced at the time that the protocol was good enough to base a company on, Vogt and Sebe say. For one, the 2013 protocol relied on injecting the progenitor cells into the brains of mice. Undifferentiated stem cells have the potential to form tumors, and research suggested that human progenitor cells, although more mature, could also cause tumors “over time,” says Stewart Anderson, director of research in child and adolescent psychiatry and behavioral services at the Children’s Hospital of Philadelphia in Pennsylvania, who is not involved with Neurona.
Because of this, the Neurona team says it decided to use cells that were committed to becoming a particular cell type in NRTX-1001; Nicholas has since called that 2013 protocol simply a “beta version” of the company’s approach. “It has no bearing on Neurona, NRTX-1001 or the ongoing trial,” he wrote in an email to Spectrum.
That protocol was the last published data that many researchers saw before Neurona officially launched and went quiet, as startups often do. The silence makes sense, Vogt says, if they want to patent the work. But it also made it difficult for outsiders to assess the company’s progress, he says. “Communication just stopped.”
T
Vogt was an investigator on the 2013 paper. He acknowledges that back then the available antibodies were not highly specific, so identifying cell types was more challenging than it is today. Somatostatin interneurons, one of the cell types that the team aimed to produce, express the protein calretinin, for example, but at a very low rate, he says. If a researcher picked up calretinin in a mouse’s brain, as was seen in the 2013 paper, “it’s more likely that a calretinin cell is going to be a VIP [vasoactive intestinal peptide-expressing] cell,” which is the kind that inhibits other inhibitory cells.
Nicholas says they never found evidence of inappropriate cell types in the batches they produced back then. VIP cells, for example, would not express the transcription factor LHX6, whereas MGE-derived cells would, he says, and VIP cells and others that originate in the CGE would have their own unique markers.

Yet it was difficult to know exactly which cells the team had in hand back then, Vogt says, which made him and some others nervous. “Are [the cells] a generic GABA cell that could influence inhibition in the brain but might also project, grow and do something else that you don’t want?” Vogt says he was not convinced that question had been resolved before the company was started.
But Nicholas felt the work sufficiently demonstrated the promise in the approach, even if it was not the product that would end up in people. “We saw the potential. We wanted to move it as quickly as we could,” he says.
Indeed, time was of the essence. The company had started out with about $100,000, raised from friends and family. By 2013, it was running out of money, Nicholas says. They were hearing “no” from potential investors and needed to show venture capitalists tangible progress if they were going to avoid shutting down entirely.
B
In 2015, he published a review with Robert Hunt, who had been a postdoctoral researcher in his lab, describing the state of the field for interneuron transplantation in the treatment of epilepsy. In it, Baraban and Hunt noted some of the limitations of the 2013 paper, including that the protocol generated some excitatory pyramidal cells that do not originate from the MGE. (Baraban and Hunt both declined to be interviewed for this article.)
Then, in a review earlier this year, Baraban and postdoctoral researcher Joseane Righes Marafiga raised further concerns with the 2013 paper, writing that “only a very small fraction” of the “putative ‘MGE-like’ cells” transplanted into a mouse’s brain survived. They also noted that the paper did not identify any parvalbumin-positive neurons, and that 73 percent expressed calretinin, the marker more common for interneurons that originate in the CGE. (Marafiga did not respond to multiple requests for comment.)
Nicholas says those criticisms stem from a misunderstanding of how human MGE progenitors develop. Parvalbumin is expressed in the human brain only after birth, he says, so “even if you culture these human cells for nine months, and you take them to a perinatal newborn stage, of course they’re not going to have parvalbumin — they’re still developing.” Because the human cells are developmentally delayed relative to mouse cells, they also have different electrophysiological properties at a given stage, making it difficult to compare across species. The calretinin, Rubenstein adds, could have been solely from somatostatin neurons, one of the inhibitory types the team was aiming to produce.
Sebe did not feel like things were so clear. As part of their previous work on the multi-lab project, she and her colleagues had spent time assessing the shape and firing properties of cells to determine if they were inhibitory interneurons. But once Nicholas took the helm, another group took charge of that vetting process. “Those of us who had done all of those recordings for so long no longer had the ability to access the cells,” Sebe says. Though she and her colleagues caught glimpses of the data in those lab meetings, they could not confirm the findings themselves, which made them uneasy.

Nicholas disputes that account and says it was the Baraban lab’s decision to stop working together. “We were sharing data,” Nicholas says. But because the data were not as expected, “they were convinced that it wasn’t worth putting effort into.” As a result, he says, “we just decided to go our separate ways.”
In 2016, Baraban founded Epygenix Therapeutics, which uses zebrafish models of epilepsy to screen drugs for the condition. He also continued to study the use of MGE tissue to treat epilepsy — no pluripotent stem cells required — which could eventually put him in competition with Neurona’s approach, Kriegstein says. Using that technique, in October 2020 Baraban and his colleagues successfully treated an epileptic sea lion by using transplanted pig MGE tissue.
Baraban’s name does not appear on Neurona’s website, except in citation. His lab motto, which serves as a banner for his X (formerly Twitter) page and features a drawing of the sea lion, reads “Hope over hype.”
I
Updated information about that mixture is detailed in the paper published today in Cell Stem Cell. Although some questions remain about how the treatment works, the paper shows that Neurona can successfully make cortical interneurons from pluripotent stem cells, says Jack Parent, professor of neurology at the University of Michigan in Ann Arbor, who was not involved in the work. None of the cells produced by its protocol express parvalbumin, which the researchers attribute to its “known postnatal expression onset,” but about one-third express somatostatin, according to the new study. And none express specific markers for CGE cells, such as SP8 or NKX2-2.
Improved screening techniques help ensure that the cells in NRTX-1001 are the right kind and in the right stage of development, Rubenstein says. Research from his lab over the past decade identified additional genes, such as MAFB, that are restricted to MGE interneurons, flagging those cells. A new antibody that binds to a protein on the surface of MGE interneurons also helps to sort the target cells, and the addition of single-nuclei RNA sequencing provides further confirmation of those cell types.
Over the past 10 years, the Neurona team also worked to figure out how to limit any remaining progenitor cells, Parent says. Neurona harvests its cells at a specific post-mitotic stage of development — early enough that the cells can still migrate and integrate, and late enough that they will not produce tumors, the new work shows. That timing hits the “sweet spot,” Kriegstein says.
In the study, the cells migrated and dispersed throughout the hippocampus when transplanted into the brains of mice that model epilepsy, and then functionally integrated into the existing circuits. Seizures decreased by at least 75 percent in 72 percent of the mice after treatment. More than half of the animals were seizure-free six months after treatment, and the effect lasted through another three months of observation.
Though Parent calls the lack of parvalbumin-positive cells identified “disappointing,” Rubenstein, for his part, is not worried about these results. “Frankly,” he says, “we don’t know what, for helping the patient, is the balance of somatostatin and parvalbumin you need.”
Another limitation of the new study is that it does not reveal the mechanism behind how the transplanted cells quell the animals’ seizures, Parent says. Future work could use optogenetic or chemogenetic approaches to silence the implanted cells “and show that they have to be active, and presumably integrated, to have the effect,” he says. Even so, he says, the work is “exciting, and it supports their clinical study.”
Vogt agrees that the paper is an improvement over 2013’s, but he remains uncertain about the types of cells present in NRTX-1001, based on the new work. The cells produced in the new study are “not a pure population of cortical interneurons,” he says. Neurona shows that it has somatostatin cells that seem to successfully limit the animals’ seizures, he says, but there is also a group of cells in the mixture that the team has yet to identify — and it is possible that they could have negative effects in the long term if those are in the clinical product. “The real takeaway for me is: What are the unknown cells, and what are they going to do?” Vogt says.
T
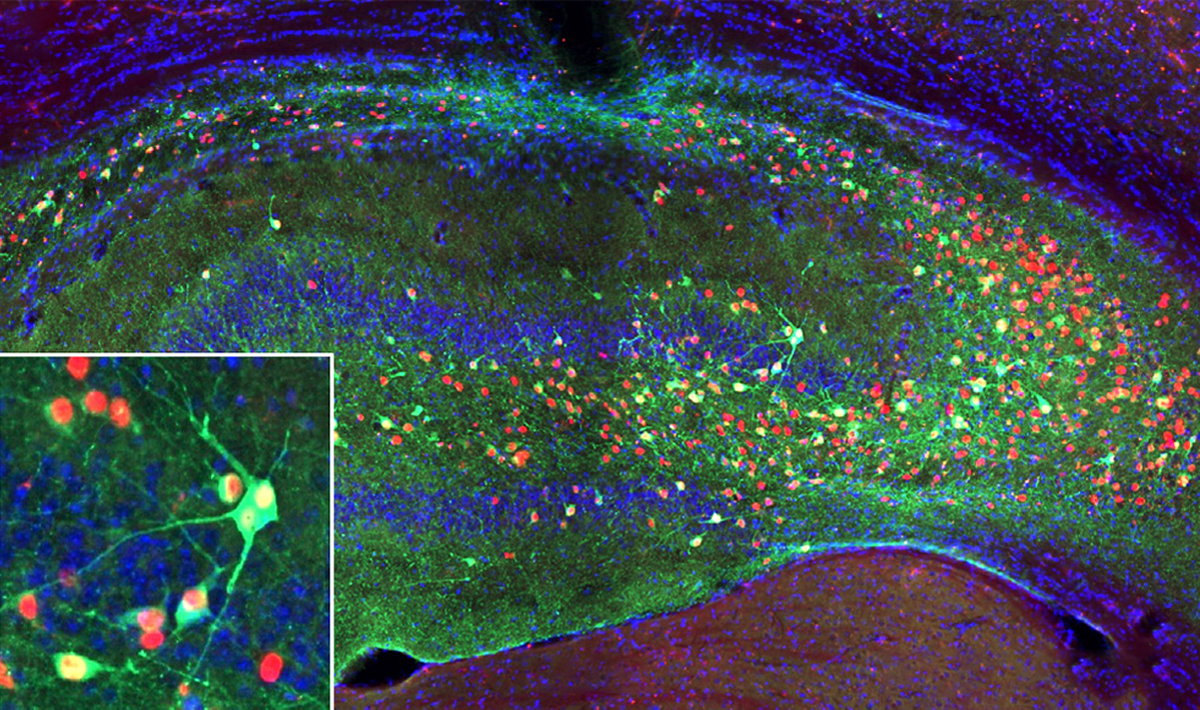
The standard course of treatment for drug-resistant epilepsy is to either implant electrodes in a person’s brain, which can sometimes zap the cells out of inducing a seizure, or to surgically slice out the problematic bit of brain tissue. Neither approach is perfectly effective, says Harish Babu, who performed Longo’s surgery, and both carry risks. Neurona’s new treatment involved an MRI-guided surgery to inject the special inhibitory cells into the brain. In the months after, Longo and Adkins went from an average of 32 and 14 seizures a month, respectively, to nearly zero.
Gordon Fishell, professor of neurobiology at Harvard Medical School, who collaborated on some of the early research but is not affiliated with Neurona, admits that he thought it was a “crazy experiment” but says he finds the results compelling. “I can’t believe this shit works,” he says.
“It’s exciting; it’s thoughtful; it’s pragmatic — I think it’s the right option” for a new treatment, says Daniel Lodge, professor of pharmacology at the University of Texas Health Science Center at San Antonio.
Still, it is a sample size of only two, Parent says. And until the participants have been tracked longer term, it is difficult to know whether the results are from the treatment itself or a side effect of the surgery, he says. “There’s literature that just breaking the blood-brain barrier has some anti-seizure properties, transiently.”
In Neurona’s new study, it took between five and seven months to eliminate seizures in most of the epileptic mice treated with NRTX-1001. “Six weeks after the implant, there’s no difference between the cell or vehicle control groups. That’s because it takes a few months for the cells to integrate and mature,” Nicholas said when he presented the data at the 2022 Epilepsy Foundation Pipeline Conference in Santa Clara, California. Longo and Adkins, on the other hand, both began improving in the first two months after being treated, which also raises questions about whether the same mechanisms are at play. One possibility, Vogt says, is that the cells release GABA in the person’s brain, which helps to quiet overexcited circuits without requiring time for the cells to integrate into them. Nicholas suggests it also may be that the mice, which are more severely affected by seizures than their human counterparts, require more time for the effect to show.
As a safety precaution, Neurona first enrolled participants like Longo and Adkins, both of whom have mesial temporal lobe epilepsy in their non-dominant hemisphere — meaning that if anything went wrong, surgeons could later remove that portion of brain tissue without causing major neurological problems. Moving forward, they plan to enroll participants with focal epilepsy on their dominant side, for whom surgery is not an option. The U.S. Food and Drug Administration approved that addition to the trial last December. Neurona is also planning to submit a protocol for a trial for people with bilateral focal epilepsy later this year. The company plans to eventually run a placebo-controlled trial of NRTX-1001.
W
If all goes well, Neurona plans to explore using its approach to treat other conditions, including Alzheimer’s disease, Parkinson’s disease, chronic pain, schizophrenia and autism. And Neurona isn’t the only one. Other companies are readying their own similar products. The biotechnology company BlueRock Therapeutics, for example, is testing a different cell-therapy approach for Parkinson’s, implanting dopaminergic neurons to replace those lost in the disease.
During this period of uncertainty, the community is rooting for the trial to succeed for the participants’ sakes, Anderson says. The challenges are very real, he says, because the stakes are high.
“We’re trying to change circuitry in the living brain of an adult human. That’s a big deal.”
Neurona’s new paper might put the company one step closer to achieving that goal, but final answers will have to wait for the clinical trial to conclude. The company’s decade of work may have paid off, says Matthew Shtrahman, assistant professor of neurosciences at the University of California, San Diego. Or maybe not.
“If it goes well, they’ll be trailblazers,” Shtrahman says. If it doesn’t, “they’ll be called cowboys. That’s the nature of the business.”
Explore more from The Transmitter
RNA drug corrects calcium signaling in chimeric model of Timothy syndrome