Courtesy of Choi-Fong Cho / Brigham & Women’s Hospital / Harvard Medical School
THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.
A new method reveals which drugs can cross the cellular barrier that separates the brain from the bloodstream1.
The blood-brain barrier protects the brain from foreign invaders. But it also blocks out many chemical compounds, creating a major hurdle for drug development. Treatments for autism and other psychiatric conditions need to cross from the blood into the brain in a reliable way.
Researchers can use synthetic systems to model the blood-brain barrier in laboratory dishes. But these models are expensive to assemble, limiting the number of drugs that researchers can screen at a time.
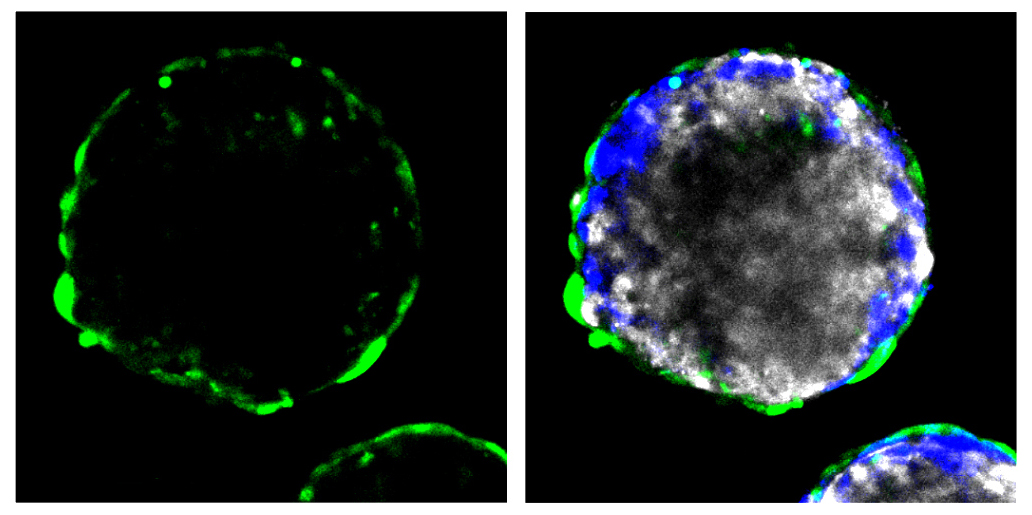
The new method offers an inexpensive way to screen several compounds at once. It involves co-culturing the three types of cells that form the blood-brain barrier: endothelial cells, which line the inside of blood vessels; pericytes, which surround the endothelial cells; and astrocytes, which support the healthy functioning of neurons. Human cell lines for all three cell types are commercially available.
After 12 hours in culture, the cells organize into tiny spheres. Endothelial cells and pericytes form the outside of the spheres, and astrocytes make up the interior.
The method was described 6 June in Nature Communications.
Test run:
Researchers exposed the spheres to a peptide known to cross the blood-brain barrier, called angiopep-2. They tagged the peptide with a fluorescent marker. When they looked under a microscope, they saw a fluorescent signal deep inside, indicating that the peptide had crossed into the sphere. Another peptide known not to cross the barrier did not permeate the spheres.
The team tested 16 fluorescently tagged peptides that were assumed to cross the blood-brain barrier based on their size, water solubility and electric charge. Only five of the peptides readily passed into the spheres. One of the five disrupted the structure of the spheres, so the researchers eliminated from it from further study.
They injected the remaining four peptides into mice and then inspected the animals’ brains for a fluorescent signal. All four peptides accumulated in the brain.
The peptide that permeated the spheres most readily was not the most abundant in mouse brain tissue. Still, the technique could help scientists narrow the search for drug candidates to test in animal models, the researchers say.
By joining the discussion, you agree to our privacy policy.