The most personalized medicine: Studying your own child’s rare condition
A handful of scientists are committed to advancing research on the autism-related genetic conditions their own children have.
The day Michael Boland’s son was born in July 2018 was blissfully normal. It had been a routine birth, and little Lukas aced the Apgar, a standard health test given to newborns. The next day, Boland made a quick trip home. As he walked back into the hospital room, he saw Lukas move in a sudden, odd way.
“What was that?” he said to his partner, Maja Horn.
“We saw him do that earlier,” Horn said.
A first-time mother, she wondered if the brief, jerky movements were typical of newborns. Or maybe it was hiccups?
Boland suspected otherwise. A cell biologist at the Institute for Genomic Medicine at Columbia University, he studies developmental and epileptic encephalopathies. He knew what seizures looked like in infants. When Lukas moved the same way again an hour later, Boland alerted the doctors. They whisked Lukas to the neonatal intensive care unit and put him on antiseizure medication. Two and a half weeks later, genetic testing revealed a mutation in a gene called STXBP1.
“Oh my God, I was devastated,” Boland says. “I had an idea what we were facing.”

He had not studied STXBP1, or syntaxin binding protein 1, but he knew that it plays a critical role in the transmission of electrical signals between neurons. Researchers had identified mutations in STXBP1 that reduce that signaling as a cause of infantile epileptic encephalopathy in 2008. Since then, increases in genetic testing have revealed STXBP1 encephalopathy in about one in 33,000 children. Clinical symptoms vary, but include epilepsy and, often, severe cognitive impairment; about 20 percent of children with the condition exhibit autism traits. Of the most affected children, Boland says, “they’re not going to be potty trained ever, they’re not going to learn to dress themselves.”
A couple of months after Lukas’s birth, Boland sat down with his colleagues at the Institute, David Goldstein and Wayne Frankel, and told them what was going on with Lukas.
“Wayne was like, ‘You’ve got to be kidding me!’” Boland remembers. “David’s jaw hit the table.”
“When do we start working on STXBP1?” Boland asked them.
“Immediately,” they responded.
With that, Boland became one of a handful of scientists in an unenviable but potentially important position: He would turn his heartbreak into hard data and study his own child’s condition. “I’m a scientist. . . This is my son. We have all of the tools here to do this,” he says. “It just feels like that’s what I was trained to do.”
In the world of rare autism-linked genetic syndromes, parents are already playing a central role, pushing to raise funds and advance investigations into their children’s conditions. “Parents are essentially kick-starting, and frankly, de-risking the research,” says Charlene Son Rigby, who in 2017 cofounded the STXBP1 Foundation, which has three parents on its science advisory board, including Boland.
Attracting parents who are also scientists to the cause only turbocharges those efforts. Nasha Fitter, a cofounder of the FOXG1 Research Foundation, a parent-led foundation for research on an autism-linked condition called FOXG1 syndrome, could hardly believe it when she stumbled on a 2017 Facebook post by FOXG1 parent Soo-Kyung Lee about a grant she and her husband, Jae Lee, both respected neuroscientists, had secured. “Hold up, you guys are parents and you’re scientists?” she remembers thinking, even before she knew of their expertise and reputation for rigor. The Lees now lead the FOXG1 Center of Excellence at the University at Buffalo in New York State and receive considerable funding from the foundation. FOXG1 families are unfortunate in many ways, Fitter says, “but we’re very fortunate with Soo and Jae.”

Experts see little risk in the personalized research of people like Boland and the Lees, noting that ethical review boards and the peer review process help protect against conflicts of interest. Meanwhile, the upside of the urgency and commitment parent-scientists bring may be considerable. “Being extra super smart is great,” says William Dobyns, a pediatric neurologist and medical geneticist at the University of Minnesota in Minneapolis who has helped identify many single-gene brain disorders, “but focus and motivation, that’s one of the difference makers. That gets progress.”
S
“Once we recognize a specific genetically defined disorder, then the possibility of developing targeted therapy is here,” Dobyns says.
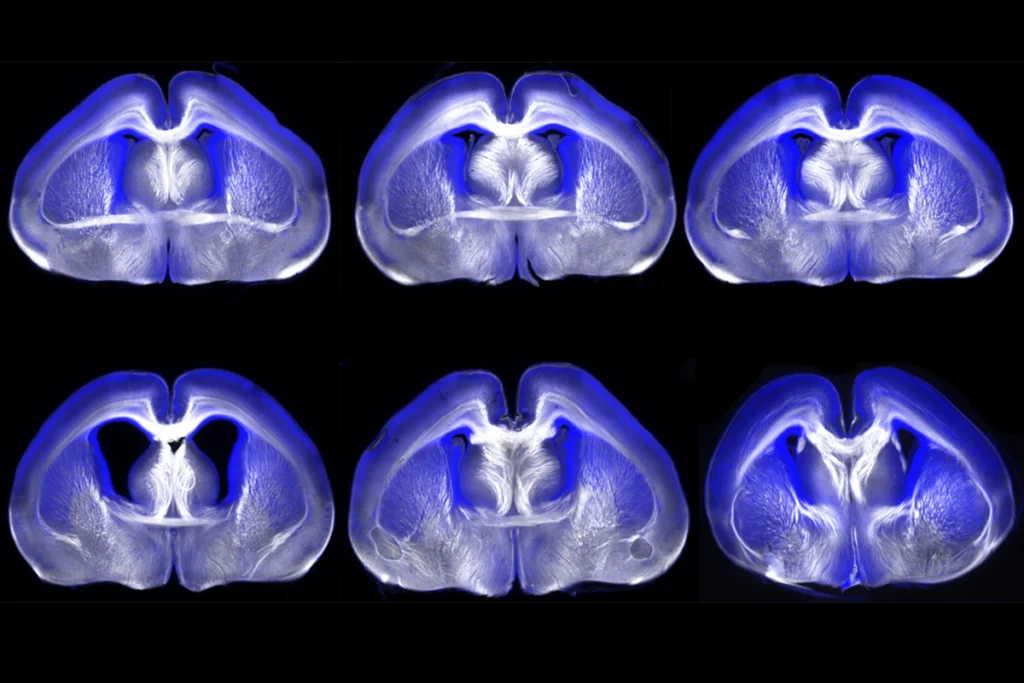
Soo Lee understood that better than most. When her daughter Yuna was born in 2010, Lee was a rising star in the world of neurodevelopmental biology. Her research focused on the role of transcription factors, which regulate genes, during brain development. The demands of her career were intense — so much so that when her infant daughter showed signs of profound developmental delays, she worried it was somehow her fault for working too much. Yuna missed every milestone, had enormous trouble feeding and sleeping and had seizures beginning soon after birth. Magnetic resonance imaging (MRI) revealed microcephaly, a small brain, but genetic testing did not initially turn up any mutations. Yuna was diagnosed with congenital Rett syndrome, a catchall for children who have clinical similarities to the autism-linked condition.
The Lees pressed to keep searching for a genetic culprit. Soo Lee took to carrying Yuna’s MRI results wherever she went, including a multiday meeting for the National Institutes of Health held in San Francisco, California. There, she talked about her then 2-year-old daughter’s condition with a researcher who offered to consult a radiologist colleague who had deep experience reading pediatric neurological MRI results. A few days later, that radiologist reported that the abnormalities in Yuna’s brain structure might be tied to FOXG1, a gene so critical to brain development that mice lacking both copies do not develop a functional brain and die shortly after birth. (The same is true of STXBP1.)
The idea that her own daughter might have a condition related to a neurodevelopmental gene, encoding a transcription factor no less — the very thing Lee studied — seemed almost too coincidental to be believed. Although FOXG1 was well known, the syndrome related to FOXG1 mutations had only been named in 2011 and wasn’t yet widely recognized. When the Lees had Yuna tested for it specifically, the radiologist was proved right. Soo Lee reviewed the raw sequencing data herself to be sure. She estimates that Yuna was the 20th child in the world to be identified with FOXG1 syndrome. There are still fewer than 1,000 known cases, although there are likely to be many more who have not been identified.
The hallmarks of the syndrome include microcephaly, cortical atrophy and weak or missing connections between brain hemispheres, as well as seizures, cognitive disabilities, absence of language, movement disorders and, sometimes, autism. Children who have a completely inactivated copy of the gene, like Yuna, have more disabling traits than those with a more mildly affected version that produces faulty FOXG1 protein.
Yuna’s diagnosis prompted Soo Lee to make FOXG1 a centerpiece of her research. “I thought, this is what I have to do,” she says. Jae Lee, who had done important work on gene regulation of metabolism, joined her. “I was more than glad to drop everything else,” he says.

Much of the Lees’ home on a quiet cul-de-sac near the university is organized with Yuna in mind. The house features wide-open spaces and hardwood floors that can be readily navigated with a wheelchair. Construction on a small indoor swimming pool is underway, because Yuna enjoyed the hotel swimming pools they frequented when they drove across the country from Oregon to Buffalo to start their center in 2019.
Small and thin for a 12-year-old, Yuna usually wears soft clothing such as sweatpants and a fleece top, with her hair pulled into a ponytail atop her head with a fuzzy scrunchie. (Her dad has gotten very good at doing her hair in the morning.) She cannot walk or speak, but her family know what she likes — including stuffed animals and toys that light up or play music. After she gets home from her specialized school, she spends a lot of time in a play area they’ve created for her in an alcove off the kitchen. Her poor motor control means that she is constantly moving, but when her caregiver puts a sticker on the couch and encourages Yuna to go get it, the girl rocks and reaches her way to the couch. The Lees credit years of therapy and hard work. Her movement has become “more purposeful because she has better control,” Jae Lee says.
They take heart from other small, hard-won changes. Yuna never used to make eye contact with her parents. Recently she began glancing out the school bus window at them as they waved goodbye in the morning. One day when Jae did not join Soo in the driveway, Yuna looked far longer than normal. Soo says she believes Yuna was searching for her father. The next day Jae was back in position and Yuna, presumably satisfied, resumed her usual glance. “She’s doing much better than what I thought [was possible] 5 years ago,” Soo Lee says. “It’s a very subtle thing. Nowadays, I can tell what she likes, that she’s happy. It’s just so much easier to know who Yuna is.”

T
The Lees have focused their efforts on mouse models. The first one they analyzed lacked one copy of the FOXG1 gene and showed altered brain structure and behavior that mimicked the movement, learning and memory deficits seen in children with FOXG1 syndrome. The Lees have since made multiple mouse models that mimic various mutations found in people. And they have shown that FOXG1 helps establish the brain’s cortical layers and create the corpus callosum, which connects the left and right brain hemispheres.
Boland, too, is working with a mouse model of STXBP1, with help from Frankel, who has decades of experience in the field. But Boland also grows human pluripotent stem cells, which he coaxes into two different models: two-dimensional neuronal networks that look like lacy latticework, and three-dimensional brain organoids, which look like chickpeas yet faithfully recapitulate the early cell growth in developing brains. He has even created models using Lukas’s cells and his own. “[That’s] a 3D model of my son’s brain in a dish,” he says during a tour of the lab. The three models — neuronal networks, organoids and mice — trade biological complexity for granularity and together, Boland says, allow more nuanced comparisons of how typical and STXBP1 neurons communicate.

Fortunately, FOXG1’s work appears to be incomplete at birth, the Lees have found, and STXBP1 is critical to how neurons communicate throughout life. That leaves open the possibility of drug treatments or gene therapies. Boland and Frankel are focusing on testing two gene therapies for STXBP1: a traditional replacement therapy that adds back a functional copy of STXBP1 and an adaptation of CRISPR technology that upregulates the gene’s expression. (That work is supported by a grant from the Simons Foundation, Spectrum’s parent organization, and Boland is a part-time consultant for the foundation.) Unpublished work in other labs has successfully stopped seizures and rescued learning and memory deficits in mice, Boland says.
The Lees are using their mice as platforms for drug screening. One therapy they tried in an unpublished experiment reversed some of the traits in FOXG1 model mice. “We wanted to confirm whether FOXG1 syndrome can be fixed,” Jae says. “The answer seems to be yes. We were just completely stunned.”
D
Each new skill — head control, sitting up, pulling up, crawling — was the work of many months or even years. Boland and Horn call them “inchstones” not milestones. Nonetheless, they say, Lukas is easygoing and engaged. He loves spinning tops, musical toys and board books. Lying on the floor with an Elmo book, he dips his head to the page and touches it with his lips, giving Elmo a kiss. Such social behavior feels like a gift, Boland says, while mashing up sweet potatoes, spinach and quinoa for Lukas’s dinner. “When he can give you those big beautiful brown eyes that stare into your soul, it makes it easier.”

During her pregnancy, Horn, who was over 40 and at increased risk for having a child with a disability, worried a little bit about that possibility. “Will you do everything you can?” she asked Boland. He said he would. But that was a hypothetical conversation, and the reality of Lukas’s condition was a shock. Over time, however, says Horn, a professor of Spanish literature at Barnard College in New York City, she has come to fully accept Lukas for who he is, and the experience of raising him has changed her “in every imaginable way.” She, too, has shifted her academic interests to think about perceptions of ability and disability. She is glad Boland is studying STXBP1 — that he is, in fact, doing everything he can. But she is not willing to try anything too risky on her child. Her focus is on cherishing Lukas as he is, “a child that’s so lovely and happy,” and facing the immediate future. “My hope is that he will be able to express his needs and wants on his [communication] device. . .to be able to say I’m hungry, I’m thirsty,” she says. “I think that’s totally within reach.”
Boland and the Lees have been changed as well, for better and for worse.
One Sunday afternoon, when Yuna was 5 years old, Soo Lee collapsed in the living room. She had developed vestibular neuritis, a destabilizing condition caused by inflammation, which Soo attributes to stress. Seven years later, she manages her condition with medication but must limit work hours, screen time and some daily activities like driving. When her 9-year-old son, Joon, “wants to show me a YouTube video, he says, ‘Wait, wait, let me lower the brightness,’” she says with a laugh.
Science is famously competitive and ego-driven; there is only so much money and recognition to go around. For Boland and the Lees, however, ego has less to do with it these days. Regardless of funding or support, Jae Lee says, “this is what we would be doing.” Interactions with other scientists are different, too. It used to be “like holding a poker hand,” Boland says. No more. “As a parent, I’m less of the poker player. I’m more like, these are my cards. If you can learn from me, then maybe that’ll help you develop a therapy faster than mine.”
Explore more from The Transmitter
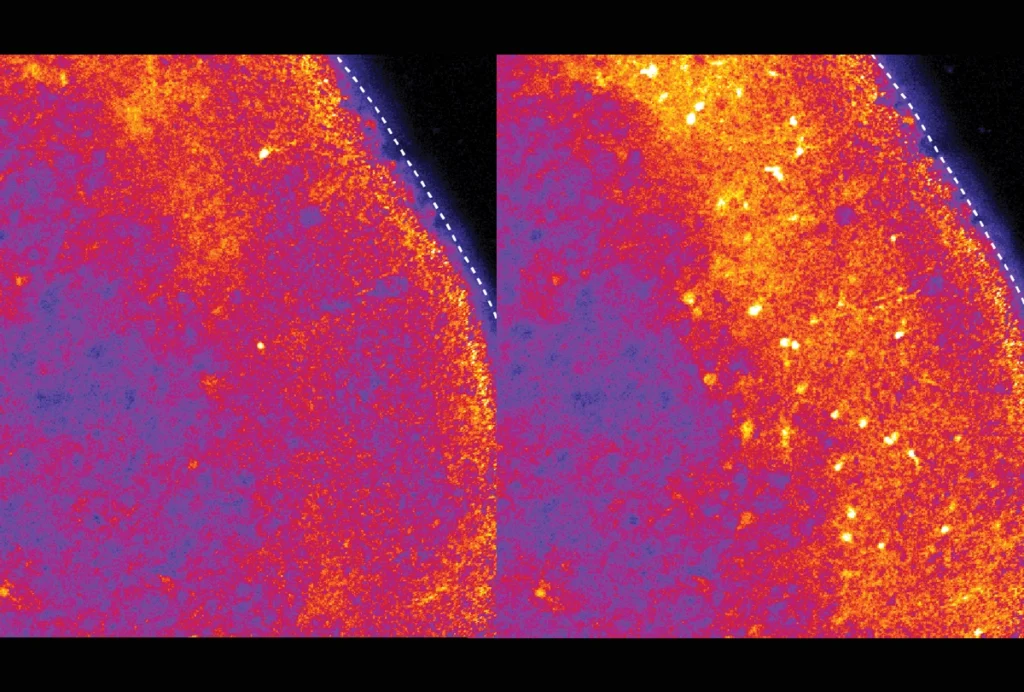
RNA drug corrects calcium signaling in chimeric model of Timothy syndrome