©istockphoto.com/Kurt Drubbel
THIS ARTICLE IS MORE THAN FIVE YEARS OLD
This article is more than five years old. Autism research — and science in general — is constantly evolving, so older articles may contain information or theories that have been reevaluated since their original publication date.
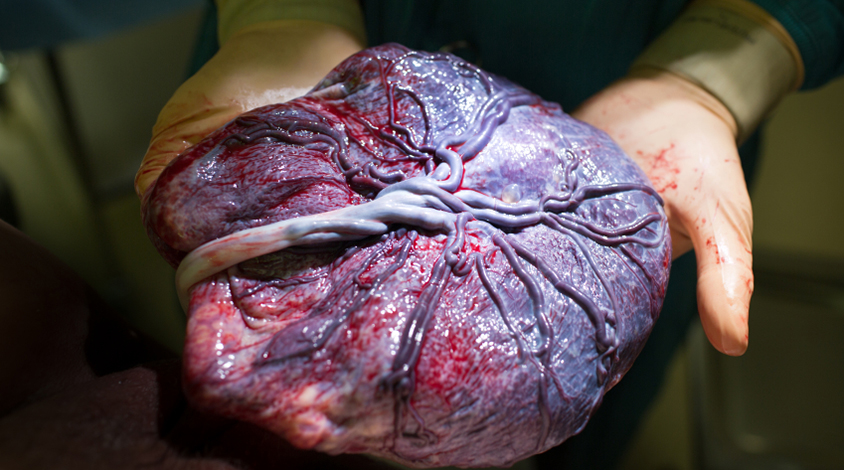
A new test claims to assess a child’s risk of autism based on abnormal folds in the placenta, and is being offered to pediatricians in the U.S. But with little evidence to support its use, experts say the test is premature and unethical.
Yale University researcher Harvey Kliman introduced the PlacentASD Test in 2013 as a tool to gauge a child’s risk of autism at birth, just months after publishing a study on placental folds.1
Already, he says, parents ship thousands of freshly delivered placentas to his lab each year, though he declines to say how many are analyzed for autism risk. Some reports indicate the test could cost upward of $2,000.
Autism experts are not impressed.
“I am truly appalled at the rush to market a test that has such weak predictive power to vulnerable families,” says Helen Tager-Flusberg, director of the Center for Autism Research Excellence at Boston University.
Researchers note that no other peer-reviewed reports have linked placental folds to childhood disorders, and none establish a firm link to autism. Kliman has also not yet confirmed autism diagnoses for children whose placentas he examined at birth.
These unknowns call the test’s predictive power — and ethics — into question, says Catherine Lord, director of the Center for Autism and the Developing Brain at New York-Presbyterian Hospital. “The idea that this is being marketed without fully acknowledging its limitations is scary,” she says.
Early signs:
Autism can be reliably diagnosed by age 2 using behavioral analyses, but scientists are trying to develop ways to detect the condition earlier to give children access to treatments sooner.
In their quest, they have sifted through data from brain scans, eye-tracking experiments, blood tests and even saliva samples for telltale differences between infants destined for an autism diagnosis and those who are not.
Some of these efforts have focused on the younger siblings of children with autism, who have a one-in-five chance of developing the condition themselves. Kliman’s study included 117 such ‘baby sibs’ and 100 infants with no family history of autism. His team examined four slices of each child’s placenta, each slice roughly the size of an adult’s thumbnail, under a microscope, looking for tiny structures in the placenta called trophoblastic inclusions. These inclusions form when cells divide too quickly and cause the placenta to fold in on itself, instead of bulging outward as it normally does.
The study found that 66 percent of the baby sibs had at least 1 inclusion, 41 percent had more than 1 and 3 percent had more than 10. Among the controls, 32 percent had at least one inclusion and 8 percent had more than one, but none had three or more.
The findings are in line with a 2007 study in which Kliman found that 39 percent of 13 preserved placentas from children with autism had the inclusions compared with 13 percent of 61 placentas from controls2.
Based on the presence of the folds, “we can very accurately say what the probability is of a child being in the [autism] risk group,” says Kliman, director of reproductive and placental research at Yale.
General marker:
However, other peer-reviewed studies, including Kliman’s own work, hint that the inclusions are not specific to autism but rather a general marker for genetic abnormalities.
For example, a 2014 study in Placenta found that the inclusions occur in 40 percent of placentas from women with a condition called placenta accreta, in which blood vessels from the placenta invade the uterine wall3. Others have linked the inclusions to a genetic condition called Maroteaux–Lamy syndrome, which can cause mild to severe developmental delays in childhood4.
Kliman himself published a brief note in a 2003 issue of Fertility and Sterility reporting that the inclusions were found in 67 percent of 48 placentas from spontaneous miscarriages resulting from genetic abnormalities5.
Even Kliman’s former collaborators are distancing themselves from the test.
“It’s one thing to believe in something with all your heart, especially for someone as smart and committed as Dr. Kliman; it’s another to be painstaking about not going beyond your data,” says Cheryl Walker, an obstetrician gynecologist at the University of California, Davis MIND Institute, who led Kliman’s 2013 study. “There are no published data to support the new test as a screening tool,” she says.
Big claims:
A brochure for the test notes that a positive result doesn’t mean a child has autism, just that the child is at increased risk. It also claims that major insurance companies, including Aetna, cover the test.
However, Aetna would not cover the test because it is too “experimental and investigational,” says Bob McDonough, senior director of clinical policy research and development for Aetna. “There are no published clinical studies examining the impact of the PlacentASD Test on clinical outcomes, and no current evidence-based guidelines of medical professional organizations or public health agencies recommending use of this test,” McDonough says.
Still, companies like Aetna might end up reimbursing for the test because the Yale Medical Group, the organization that oversees Kliman’s clinical practice, bills for each incremental task, such as fixing a sliver of placenta onto a pathology slide, rather than for the test itself.
Tests developed by individual labs can be sold without federal review or regulation, a fact that families may not realize, says Eric Pahon, a spokesperson for the U.S. Food and Drug Administration’s (FDA’s) Center for Drug Evaluation and Research. (Pahon declined to comment on Kliman’s test because it is not registered with the agency.)
“The brochure is aimed directly at parents who could not possibly realize how limited the evidence actually is for this biomarker,” says Tager-Flusberg. “Parents would believe that this test has been vetted by the FDA or equivalent institution. It is shocking that Dr. Kliman has begun this advertising campaign with the full weight of Yale University behind his venture.”
Bottom line:
In the meantime, Kliman says he has tracked a small group of children since birth and has found that only children with at least three inclusions show developmental delays during their first 2 years. These children received some form of early treatment, and a “significant number” of them caught up to their typical peers by age 2, showing no evidence of autism or delays, he says.
“So that, on a very small scale, has really encouraged me in terms of what this test does,” Kliman says. “So far, we’re on track for the test to be working.”
The results are unpublished, and Kliman declined to provide details about the number of children, or the diagnostic tools or types of interventions used.
But it may be difficult for Kliman to prove that the children who improved ever had autism, as the condition cannot be reliably diagnosed until age 2, says Inge-Marie Eigsti, associate professor of psychology at the University of Connecticut, who has not seen Kliman’s new data. Eigsti studies children who eventually lose their autism diagnosis, a so-called ‘optimal outcome,’ and notes that many baby sibs without autism show delays in their first year but later catch up to their peers.
“The bottom line is that it would be wonderful to have a diagnostic test based on placental markers, but I think this is way ahead of where it needs to be,” says Arthur Caplan, director of medical ethics at New York University. “You absolutely have to subject what you claim to be true to peer review before you recommend it or try to get someone to pay for it.”
By joining the discussion, you agree to our privacy policy.