‘Mind-blowing’ study upends conventional wisdom on oxytocin
CRISPR-edited prairie voles that lack receptors for the so-called “social hormone” still bond with their mate and pups, raising questions about the molecule’s role.
Prairie voles engineered to carry faulty receptors for oxytocin still form pair bonds and nurture their young, according to a study published today in Neuron. The findings — the culmination of 13 years of inquiry — call into question the hormone’s long-presumed critical role in promoting social behavior, the researchers say.
“We need a rethink on the centrality of oxytocin signaling,” says co-lead researcher Nirao Shah, professor of psychiatry and behavioral sciences at Stanford University in California.
Oxytocin (and the related neuropeptide vasopressin) rose to prominence in the 1990s and early 2000s through research on prairie voles — rodents that form strong social bonds with their partners and pups. Compared with the more promiscuous meadow and montane voles, the monogamous prairie and pine voles have a higher density of oxytocin binding in specific brain regions, including the nucleus accumbens. Boosting the expression of the receptors there increases pair-bonding behaviors in prairie voles, whereas blocking it limits those behaviors, according to previous studies.
The findings laid the groundwork for clinical trials of oxytocin to boost social functioning in autistic people, which have been equivocal. The new study may point to why, Shah and his colleagues say. When they engineered three lines of prairie voles to carry mutations in the oxytocin receptor gene, none of the animals showed a change in their pair-bonding or parental behaviors — suggesting that pathways other than oxytocin signaling serve to strengthen social bonds.
“It’s kind of mind-blowing, in a way,” says Larry Young, director of the Center for Translational Social Neuroscience at Emory University in Atlanta, Georgia, who pioneered some of the early studies of oxytocin in voles but was not involved in the new work.
The new findings do not invalidate past work showing that oxytocin is critical for pair bonding, or its potential for treating autism, he says, but they do suggest that animals born without the capacity for oxytocin signaling compensate with other mechanisms.
T
The professors leading that undergraduate course expressed skepticism that a single neuropeptide such as oxytocin could wield such control over something as complex as mammalian social behavior, and they stressed the importance of using complementary approaches for testing the hypothesis, Shah says — ideas that eventually shaped the focus of his own lab. He began developing tools to study how genetic changes affect prairie voles’ social behaviors. After the advent of CRISPR gene editing in 2012, he and his colleagues adapted the tool for use in prairie voles and inserted three different mutations individually into the animals’ oxytocin receptor gene to disrupt its function.
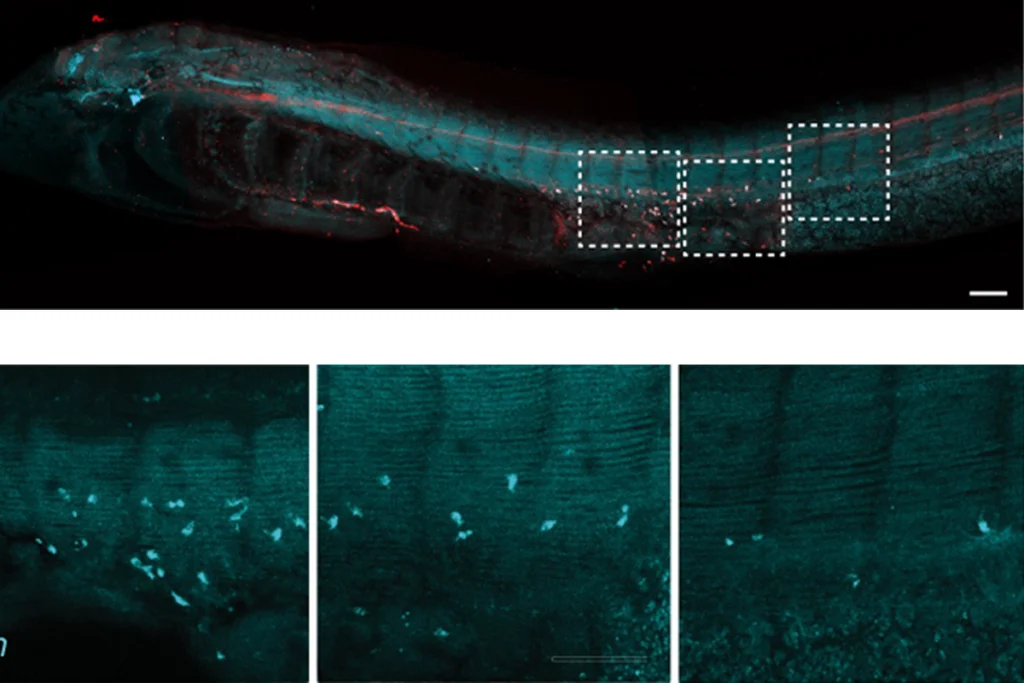
The edited voles had no discernible oxytocin binding throughout the brain, postmortem analyses of the animals’ brain tissue revealed. Yet the voles still spent typical amounts of time huddling with their partners and acted aggressively toward other members of the opposite sex — both measures of their pair-bondedness.
Mice lacking oxytocin receptors do not retrieve and return straying pups to their nest, a past study showed. But Shah’s edited prairie voles kept their pups close and groomed them just as much as wildtype animals do. Some of the female animals did have trouble nursing their young, however, suggesting that oxytocin signaling is important for that particular behavior.
But for the other social functions thought to rely on oxytocin signaling, the results suggest that “it ain’t that,” Shah says.
One caveat is that the three lines of mutants, though similar to wildtype animals in their pair-bonding behaviors, do show some individual differences, says Linmarie Sikich, associate consulting professor of psychiatry and behavioral sciences at Duke University in Durham, North Carolina, who was not involved in the study. That suggests that the engineered mutations may not fully block oxytocin signaling, she says, or that they lead to other differences between the groups.
But the lack of oxytocin binding in the animals’ brains does suggest that the mutations cause a loss of function, Shah says. He and his colleagues theorize that the differences may stem from the natural variation that exists in these complex behaviors, due to the fact that the voles are bred from wild animals. “So even though they all harbor mutant oxytocin receptors, other genetic background components alter their behaviors subtly” and could contribute to those differences, he says.
M
The new findings highlight the importance of understanding how genetic changes affect the brain from its earliest moments, says co-lead researcher Devanand Manoli, assistant professor of psychiatry at the University of California, San Francisco. And the CRISPR prairie voles enable researchers to ask, “What is happening in the context of different clinical populations?” he says.
For now, the implications for clinical trials of oxytocin are unclear.
The new results suggest that oxytocin receptors are not the ideal target to manipulate social behaviors overall, Manoli says. But because “there’s no evidence that people with autism are born without oxytocin receptors,” Young disagrees and says that past studies in adult animals born with intact oxytocin receptors showed “that oxytocin receptor signaling is critical for social learning and pair bonding.”
Young likens having oxytocin receptors from birth to an orchestra that always practiced with a conductor: Without one, they may struggle to play together. A street band, on the other hand, that never learned to rely on a conductor, can get along fine without one — like the CRISPR-edited prairie voles, it finds a way to compensate.
Manoli and Shah plan to continue studying the engineered voles to explore what that compensation may be. But they’re not convinced that the orchestra requires a conductor at all.
Early on, Shah says, “there were no conductors. People just read the scores and played them.” Regardless, he says, “it could be that there are multiple players, and oxytocin is one of them.”
Explore more from The Transmitter

$278 million cut in BRAIN Initiative funding leaves neuroscientists in limbo

Reporting bias widespread in early-childhood autism intervention trials